Page 10 - Roche Hemlibra Non-inhibitors - Product Monograph
P. 10
4. CLINICAL MANAGEMENT OF THE DISEASE OR HEALTH CONDITION
4.1. UNMET MEDICAL NEED
Replacement therapy with recombinant FVIII:
01 02
Recombinant FVIII is a widely Limitations of recombinant VIII are:
used treatment for patients • Inadequate efficacy due to incomplete
with hemophilia A with and coverage
without inhibitors to FVIII • High treatment burden and low patient
(Castaman and Linari. 2018) compliance (Castaman and Linari. 2018)
Given these challenges, there is need for therapeutics with the following characteristics:
Reliable efficacy with
an extended half life to A different route of
reduce the number of administration
administrations needed
Improved
Less treatment compliance and
burden leading effectiveness in
preventing bleeding
Safe and efficacious Improvement in
prophylactic treatment patients HRQoL
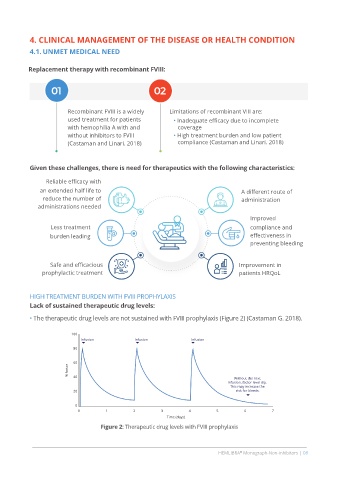
HIGH TREATMENT BURDEN WITH FVIII PROPHYLAXIS
Lack of sustained therapeutic drug levels:
• The therapeutic drug levels are not sustained with FVIII prophylaxis (Figure 2) (Castaman G. 2018).
100
Infusion Infusion Infusion
80
60
% factor 40
Without the next
infusion, factor level dip.
This may increase the
20 risk for bleeds.
0
0 1 2 3 4 5 6 7
Time (days)
Figure 2: Therapeutic drug levels with FVIII prophylaxis
HEMLIBRA Monograph-Non-inhibitors | 08
®