Page 31 - Roche Hemlibra Non-inhibitors - Product Monograph
P. 31
• For the ‘physical health’ score subscale, a ≥10-point change from baseline was considered
clinically meaningful
• The proportion of missed work days was also collected
• The schedule of assessments for the Haem-A-QoL and the missed work day item for HAVEN 3
and HAVEN 4 (expansion cohort only) included: Weeks 1, 13, 25, 49, 61 (HAVEN 4 only), 73
(HAVEN 3 only), and study completion
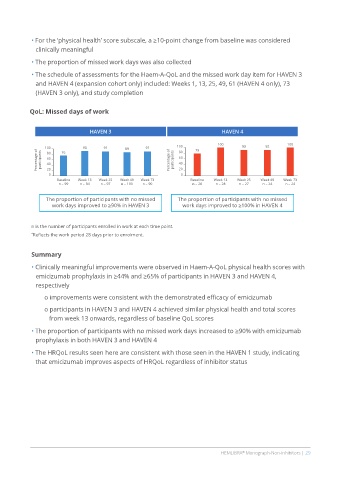
QoL: Missed days of work
HAVEN 3 HAVEN 4
100 76 93 91 89 91 100 79 100 93 92 100
Percentage of participants 60 Percentage of participants 60
80
80
40
40
20
0 20 0
Baseline Week 13 Week 25 Week 49 Week 73 Baseline Week 13 Week 25 Week 49 Week 73
n = 99 n = 94 n = 97 n = 100 n = 90 n = 28 n = 28 n = 27 n = 24 n = 24
The proportion of participants with no missed The proportion of participants with no missed
work days improved to ≥90% in HAVEN 3 work days improved to ≥100% in HAVEN 4
n is the number of participants enrolled in work at each time point.
* Reflects the work period 28 days prior to enrolment.
Summary
• Clinically meaningful improvements were observed in Haem-A-QoL physical health scores with
emicizumab prophylaxis in ≥44% and ≥65% of participants in HAVEN 3 and HAVEN 4,
respectively
o improvements were consistent with the demonstrated efficacy of emicizumab
o participants in HAVEN 3 and HAVEN 4 achieved similar physical health and total scores
from week 13 onwards, regardless of baseline QoL scores
• The proportion of participants with no missed work days increased to ≥90% with emicizumab
prophylaxis in both HAVEN 3 and HAVEN 4
• The HRQoL results seen here are consistent with those seen in the HAVEN 1 study, indicating
that emicizumab improves aspects of HRQoL regardless of inhibitor status
HEMLIBRA Monograph-Non-inhibitors | 29
®