Page 28 - Roche Hemlibra Non-inhibitors - Product Monograph
P. 28
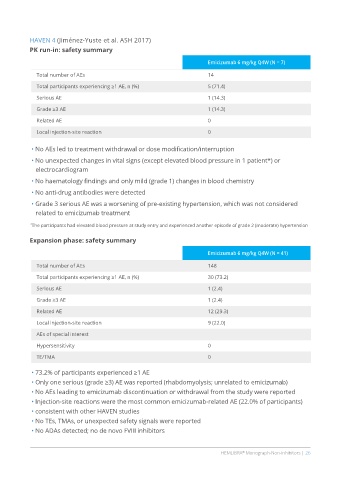
HAVEN 4 (Jiménez-Yuste et al. ASH 2017)
PK run-in: safety summary
Emicizumab 6 mg/kg Q4W (N = 7)
Total number of AEs 14
Total participants experiencing ≥1 AE, n (%) 5 (71.4)
Serious AE 1 (14.3)
Grade ≥3 AE 1 (14.3)
Related AE 0
Local injection-site reaction 0
• No AEs led to treatment withdrawal or dose modification/interruption
• No unexpected changes in vital signs (except elevated blood pressure in 1 patient*) or
electrocardiogram
• No haematology findings and only mild (grade 1) changes in blood chemistry
• No anti-drug antibodies were detected
• Grade 3 serious AE was a worsening of pre-existing hypertension, which was not considered
related to emicizumab treatment
* The participants had elevated blood pressure at study entry and experienced another episode of grade 2 (moderate) hypertension
Expansion phase: safety summary
Emicizumab 6 mg/kg Q4W (N = 41)
Total number of AEs 148
Total participants experiencing ≥1 AE, n (%) 30 (73.2)
Serious AE 1 (2.4)
Grade ≥3 AE 1 (2.4)
Related AE 12 (29.3)
Local injection-site reaction 9 (22.0)
AEs of special interest
Hypersensitivity 0
TE/TMA 0
• 73.2% of participants experienced ≥1 AE
• Only one serious (grade ≥3) AE was reported (rhabdomyolysis; unrelated to emicizumab)
• No AEs leading to emicizumab discontinuation or withdrawal from the study were reported
• Injection-site reactions were the most common emicizumab-related AE (22.0% of participants)
• consistent with other HAVEN studies
• No TEs, TMAs, or unexpected safety signals were reported
• No ADAs detected; no de novo FVIII inhibitors
HEMLIBRA Monograph-Non-inhibitors | 26
®