Page 23 - Roche Hemlibra Non-inhibitors - Product Monograph
P. 23
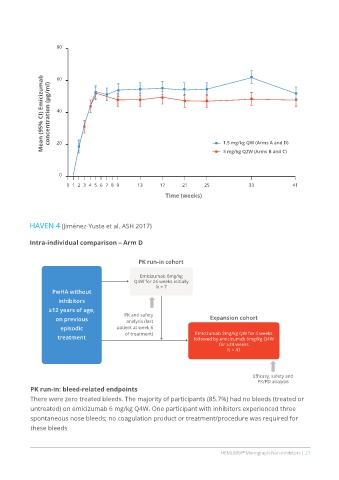
80
Mean (95% CI) Emicizumab concentration (µg/ml) 40 1.5 mg/kg QW (Arms A and D)
60
20
0 3 mg/kg Q2W (Arms B and C)
0 1 2 3 4 5 6 7 8 9 13 17 21 25 33 41
Time (weeks)
HAVEN 4 (Jiménez-Yuste et al. ASH 2017)
Intra-individual comparison – Arm D
PK run-in cohort
Emicizumab 6mg/kg
Q4W for 26 weeks initially
N = 7
PwHA without
inhibitors
≥12 years of age,
PK and safety
on previous analysis (last Expansion cohort
episodic patient at week 6
of treatment) Emicizumab 3mg/kg QW for 4 weeks
treatment followed by emicizumab 6mg/kg Q4W
for ≥24 weeks
N = 41
Efficasy, safety and
PK/PD analysis
PK run-in: bleed-related endpoints
There were zero treated bleeds. The majority of participants (85.7%) had no bleeds (treated or
untreated) on emicizumab 6 mg/kg Q4W. One participant with inhibitors experienced three
spontaneous nose bleeds; no coagulation product or treatment/procedure was required for
these bleeds
HEMLIBRA Monograph-Non-inhibitors | 21
®