Page 24 - Roche Hemlibra Non-inhibitors - Product Monograph
P. 24
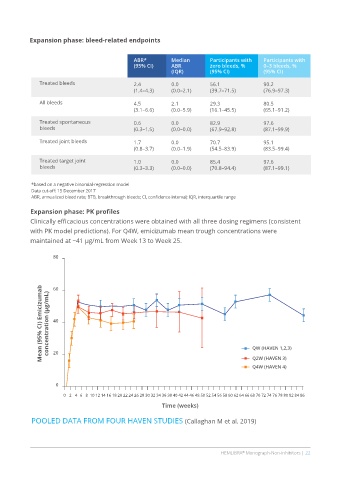
Expansion phase: bleed-related endpoints
ABR* Median Participants with Participants with
(95% CI) ABR zero bleeds, % 0–3 bleeds, %
(IQR) (95% CI) (95% CI)
Treated bleeds 2.4 0.0 56.1 90.2
(1.4–4.3) (0.0–2.1) (39.7–71.5) (76.9–97.3)
All bleeds 4.5 2.1 29.3 80.5
(3.1–6.6) (0.0–5.9) (16.1–45.5) (65.1–91.2)
Treated spontaneous 0.6 0.0 82.9 97.6
bleeds (0.3–1.5) (0.0–0.0) (67.9–92.8) (87.1–99.9)
Treated joint bleeds 1.7 0.0 70.7 95.1
(0.8–3.7) (0.0–1.9) (54.5–83.9) (83.5–99.4)
Treated target joint 1.0 0.0 85.4 97.6
bleeds (0.3–3.3) (0.0–0.0) (70.8–94.4) (87.1–99.1)
*based on a negative binomial-regression model
Data cut-off: 15 December 2017
ABR, annualized bleed rate; BTB, breakthrough bleeds; CI, confidence interval; IQR, interquartile range
Expansion phase: PK profiles
Clinically efficacious concentrations were obtained with all three dosing regimens (consistent
with PK model predictions). For Q4W, emicizumab mean trough concentrations were
maintained at ~41 µg/mL from Week 13 to Week 25.
80
Mean (95% CI) Emicizumab concentration (µg/mL) 40
60
QW (HAVEN 1,2,3)
20
Q2W (HAVEN 3)
Q4W (HAVEN 4)
0
0 2 4 6 8 10 12 14 16 18 20 22 24 26 28 30 32 34 36 38 40 42 44 46 48 50 52 54 56 58 60 62 64 66 68 70 72 74 76 78 80 82 84 86
Time (weeks)
POOLED DATA FROM FOUR HAVEN STUDIES (Callaghan M et al. 2019)
HEMLIBRA Monograph-Non-inhibitors | 22
®