Page 19 - Roche Hemlibra Non-inhibitors - Product Monograph
P. 19
7. CLINICAL EFFECTIVENESS, EFFICACY, AND SAFETY OF HEMLIBRA ®
7.1. HAVEN CLINICAL TRIAL PROGRAMME
The HAVEN clinical trial programme by Roche is one of the largest pivotal clinical trial
programmes in hemophilia. It is designed to assess the efficacy and safety of HEMLIBRA in
®
people with and without FVIII inhibitors. The programme also assesses the potential of
HEMLIBRA to help overcome current clinical challenges:
®
• the short-lasting effects of existing treatments
• the development of FVIII inhibitors
• the need for frequent venous access (Roche release. 2019)
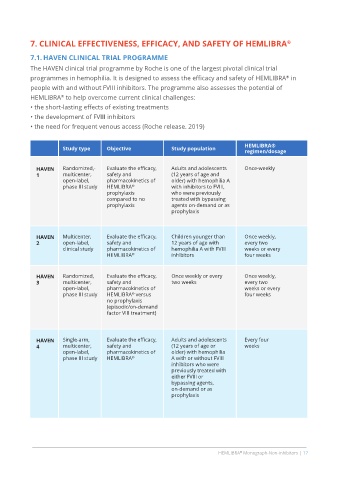
Study type Objective Study population HEMLIBRA®
regimen/dosage
HAVEN Randomized,- Evaluate the efficacy, Adults and adolescents Once-weekly
1 multicenter, safety and (12 years of age and
open-label, pharmacokinetics of older) with hemophilia A
phase III study HEMLIBRA with inhibitors to FVIII,
®
prophylaxis who were previously
compared to no treated with bypassing
prophylaxis agents on-demand or as
prophylaxis
HAVEN Multicenter, Evaluate the efficacy, Children younger than Once weekly,
2 open-label, safety and 12 years of age with every two
clinical study pharmacokinetics of hemophilia A with FVIII weeks or every
HEMLIBRA ® inhibitors four weeks
HAVEN Randomized, Evaluate the efficacy, Once weekly or every Once weekly,
3 multicenter, safety and two weeks every two
open-label, pharmacokinetics of weeks or every
phase III study HEMLIBRA versus four weeks
®
no prophylaxis
(episodic/on-demand
factor VIII treatment)
HAVEN Single-arm, Evaluate the efficacy, Adults and adolescents Every four
4 multicenter, safety and (12 years of age or weeks
open-label, pharmacokinetics of older) with hemophilia
phase III study HEMLIBRA ® A with or without FVIII
inhibitors who were
previously treated with
either FVIII or
bypassing agents,
on-demand or as
prophylaxis
HEMLIBRA Monograph-Non-inhibitors | 17
®