Page 22 - Roche Hemlibra Non-inhibitors - Product Monograph
P. 22
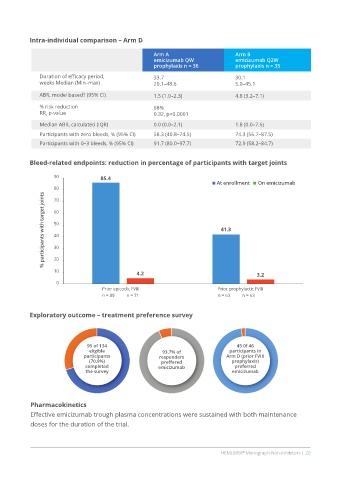
Intra-individual comparison – Arm D
Arm A Arm B
emicizumab QW emicizumab Q2W
prophylaxis n = 36 prophylaxis n = 35
Duration of efficacy period, 33.7 30.1
weeks Median (Min–max) 20.1–48.6 5.0–45.1
ABR, model based† (95% CI) 1.5 (1.0–2.3) 4.8 (3.2–7.1)
% risk reduction 68%
RR, p-value 0.32, p<0.0001
Median ABR, calculated (IQR) 0.0 (0.0–2.1) 1.8 (0.0–7.6)
Participants with zero bleeds, % (95% CI) 58.3 (40.8–74.5) 74.3 (56.7–87.5)
Participants with 0–3 bleeds, % (95% CI) 91.7 (80.0–97.7) 72.9 (58.2–84.7)
Bleed-related endpoints: reduction in percentage of participants with target joints
90 85.4
At enrollment On emicizumab
80
% participants with target joints 60 41.3
70
50
40
30
20
10 4.2 3.2
0
Prior epicodic FVIII Prior prophylactic FVIII
n = 89 n = 71 n = 63 n = 63
Exploratory outcome – treatment preference survey
95 of 134 45 0f 46
eligible 93.7% of participants in
participants responders Arm D (prior FVIII
(70.9%) preffered prophylaxis)
completed emicizumab preferred
the survey emicizumab
Pharmacokinetics
Effective emicizumab trough plasma concentrations were sustained with both maintenance
doses for the duration of the trial.
HEMLIBRA Monograph-Non-inhibitors | 20
®