Page 21 - Roche Hemlibra Non-inhibitors - Product Monograph
P. 21
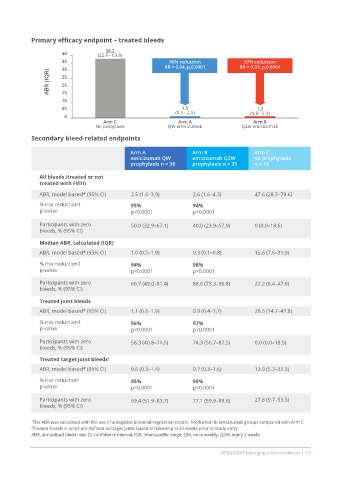
Primary efficacy endpoint – treated bleeds
38.2
40 (22.9 - 63.8)
35 96% reduction 97% reduction
30 RR = 0.04, p,0.0001 RR = 0.03, p,0.0001
ABR (IQR) 25
20
15
10
05 1.5 1.3
0 (0.9 - 2.5) (0.8 - 2.3)
Arm C Arm A Arm B
No propylaxis QW emicizumab Q2w emicizumab
Secondary bleed-related endpoints
Arm A Arm B Arm C
emicizumab QW emicizumab Q2W no prophylaxis
prophylaxis n = 36 prophylaxis n = 35 n = 18
All bleeds (treated or not
treated with FVIII)
ABR, model based* (95% CI) 2.5 (1.6–3.9) 2.6 (1.6–4.3) 47.6 (28.5–79.6)
% risk reduction† 95% 94%
p-value p<0.0001 p<0.0001
Participants with zero 50.0 (32.9–67.1) 40.0 (23.9–57.9) 0 (0.0–18.5)
bleeds, % (95% CI)
Median ABR, calculated (IQR)
ABR, model based* (95% CI) 1.0 (0.5–1.9) 0.3 (0.1–0.8) 15.6 (7.6–31.9)
% risk reduction† 94% 98%
p-value p<0.0001 p<0.0001
Participants with zero 66.7 (49.0–81.4) 88.6 (73.3–96.8) 22.2 (6.4–47.6)
bleeds, % (95% CI)
Treated joint bleeds
ABR, model based* (95% CI) 1.1 (0.6–1.9) 0.9 (0.4–1.7) 26.5 (14.7–47.8)
% risk reduction† 96% 97%
p-value p<0.0001 p<0.0001
Participants with zero 58.3 (40.8–74.5) 74.3 (56.7–87.5) 0.0 (0.0–18.5)
bleeds, % (95% CI)
Treated target joint bleeds ‡
ABR, model based* (95% CI) 0.6 (0.3–1.4) 0.7 (0.3–1.6) 13.0 (5.2–32.3)
% risk reduction † 95% 95%
p-value p<0.0001 p<0.0001
Participants with zero 69.4 (51.9–83.7) 77.1 (59.9–89.6) 27.8 (9.7–53.5)
bleeds, % (95% CI)
* The ABR was calculated with the use of a negative binomial-regression model; †Reduction in emicizumab groups compared with Arm C
‡ Treated bleeds in joints pre-defined as target joints based on bleeding in 24 weeks prior to study entry
ABR, annualised bleed rate; CI, confidence interval; IQR, interquartile range; QW, once weekly; Q2W, every 2 weeks
HEMLIBRA Monograph-Non-inhibitors | 19
®