Page 27 - Roche Hemlibra Non-inhibitors - Product Monograph
P. 27
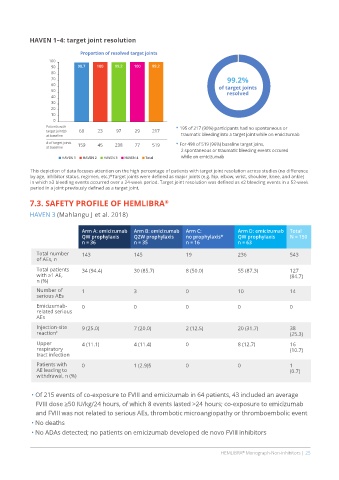
HAVEN 1–4: target joint resolution
Proportion of resolved target joints
100
90 98.7 100 99.2 100 99.2
80
70 99.2%
60 of target joints
50 resolved
40
30
20
10
0
Patients with • 195 of 217 (90%) participants had no spontaneous or
target joint(s) 68 23 97 29 217
at baseline traumatic bleeding into a target joint while on emicizumab
# of target joints 159 45 238 77 519 • For 498 of 519 (96%) baseline target joins,
at baseline
2 spontaneous or traumatic bleeding events occured
HAVEN 1 HAVEN 2 HAVEN 3 HAVEN 4 Total while on emicizumab
This depiction of data focuses attention on the high percentage of patients with target joint resolution across studies (no difference
by age, inhibitor status, regimen, etc.)*Target joints were defined as major joints (e.g. hip, elbow, wrist, shoulder, knee, and ankle)
in which ≥3 bleeding events occurred over a 24-week period. Target joint resolution was defined as ≤2 bleeding events in a 52-week
period in a joint previously defined as a target joint.
7.3. SAFETY PROFILE OF HEMLIBRA ®
HAVEN 3 (Mahlangu J et al. 2018)
Arm A: emicizumab Arm B: emicizumab Arm C: Arm D: emicizumab Total
QW prophylaxis Q2W prophylaxis no prophylaxis* QW prophylaxis N = 150
n = 36 n = 35 n = 16 n = 63
Total number 143 145 19 236 543
of AEs, n
Total patients 34 (94.4) 30 (85.7) 8 (50.0) 55 (87.3) 127
with ≥1 AE, (84.7)
n (%)
Number of 1 3 0 10 14
serious AEs
Emicizumab- 0 0 0 0 0
related serious
AEs
Injection-site 9 (25.0) 7 (20.0) 2 (12.5) 20 (31.7) 38
reaction ‡ (25.3)
Upper 4 (11.1) 4 (11.4) 0 8 (12.7) 16
respiratory (10.7)
tract infection
Patients with 0 1 (2.9)§ 0 0 1
AE leading to (0.7)
withdrawal, n (%)
• Of 215 events of co-exposure to FVIII and emicizumab in 64 patients, 43 included an average
FVIII dose ≥50 IU/kg/24 hours, of which 8 events lasted >24 hours; co-exposure to emicizumab
and FVIII was not related to serious AEs, thrombotic microangiopathy or thromboembolic event
• No deaths
• No ADAs detected; no patients on emicizumab developed de novo FVIII inhibitors
HEMLIBRA Monograph-Non-inhibitors | 25
®